SMART INNOVATION
WEBZINE2024 Vol.07, No.2
Featured
Temperature Swing Solvent Extraction
Hypersaline brines are of growing environmental concern
-
- Hypersaline brines are of growing environmental importance, but are technologically-underserved by present desalination methods. Prominent examples of such high-salinity brines include produced water from the oil and gas industry, inland desalination concentrate, landfill leachate, and flue gas desulfurization wastewater (Figure 1). Reverse osmosis (RO) is the most energy-efficient and cost-effective technique for desalinating seawater. However, because osmotic pressure scales with TDS concentration, exceedingly high operating pressures needed to overcome the osmotic pressure of hypersaline brines preclude the application of RO. Evaporation-based thermal methods, e.g., multiple effect distillation, thermal brine concentrator, and crystallizer, are the prevailing processes to desalinate or dewater highly concentrated brines. However, because the enthalpy of vaporization for water is huge (≈630 kWh/m3), these evaporative phase-change methods are inherently very energy intensive, even though the quality of energy is lower. Therefore, there is a pressing need to develop energy-efficient technologies for the more sustainable desalination of environmentally-relevant hypersaline streams.

Figure 1. Examples of hypersaline brine sources
-
- Temperature swing solvent extraction (TSSE) is a membrane-less and non-evaporative desalination technology that utilizes low-grade heat and a low-polarity solvent with temperature-dependent water solubility for the selective extraction of water over salt from saline feeds. TSSE desalination is not restricted by feedwater salinity, unlike membrane-based reverse osmosis (RO) with hydraulic pressure (or, equivalently, osmotic pressure) limitation. Because TSSE desalination does not require a phase change of water, the high energetic penalty of the vaporization enthalpy is inventively sidestepped and significantly higher energy efficiencies are attainable, specifically for hypersaline brines.
- TSSE employs a low-polarity solvent with temperature-dependent water solubility, and the working principles are depicted in Figure 2. Firstly, saline feedwater is combined with the solvent (step I), with both at temperature TL. Due to the low polarity of the solvent, the two liquids are immiscible and a biphasic mixture is formed. However, some water from the aqueous phase partitions into the solvent phase because of hydrophilic moieties on the solvent chemical structure. That is, the solvent extracts water from the saline feedwater, leaving a concentrated raffinate, whereas ionic salt species do not favor partitioning into the low-polarity solvent. The dewatered concentrate is physically separated (step II), and the water-in-solvent extract is brought to temperature TH, with moderate ΔT of ≈20-60 °C. Because the solubility of water in the solvent decreases with increasing temperatures, the temperature swing from TL → TH causes water to demix from the solvent to form a biphasic aqueous-solvent mixture (step III). The product water, containing trace amount of salt, is easily decanted from the solvent as the two liquids are immiscible and the regenerated solvent is cycled back for reuse after returning to temperature TL (step IV).
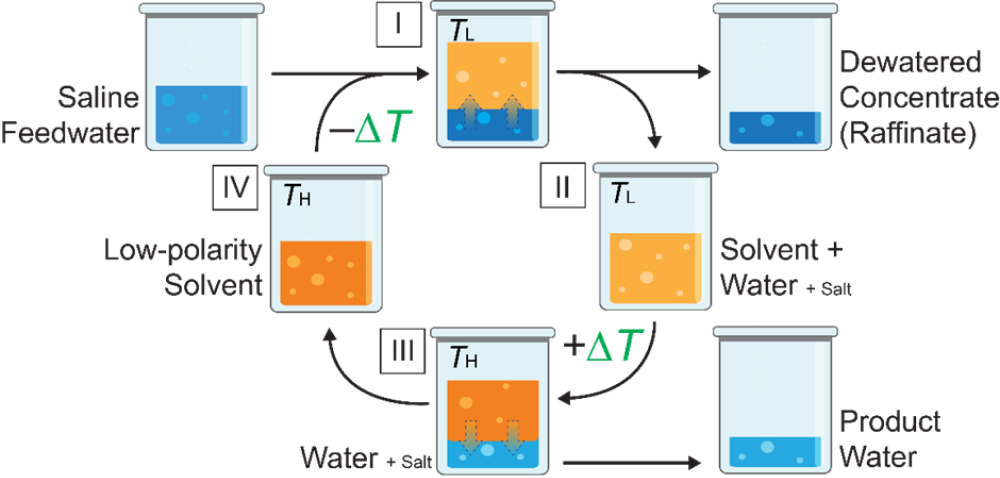
Figure 2. Schematic illustrating the working principles of temperature swing solvent extraction.
-
- TSSE desalination of hypersaline brines was evaluated using NaCl solution with concentrations ranging from 1.0 to 4.0 M, which correspond to TDS of ≈58,500-234,000 ppm. Water extraction efficiencies of three amine solvents, i.e., DIPA, ECHA, and DMCHA, are presented in Figure 3A. For every mole of DIPA, DMCHA, and ECHA, approximately 1.13, 1.00, and 0.75 moles of water were extracted, respectively, from 1.0 M NaCl brine. Critically, the three amine solvents investigated produced water even from 4.0 M NaCl with TDS concentration >6x seawater, demonstrating the promise of TSSE for ultrahigh-salinity desalination.
- Product water quality of TSSE desalination was evaluated for salt (NaCl) removal and residual solvent concentration (Figure 3B). Salt removal, SR, is defined as the percentage of NaCl removed from the product water relative to the saline feedwater, i.e., SR = 1 − Cp/Cf, where C is concentration and subscripts P and F denote product and feed, respectively). High salt removals >90% were achieved with all solvents for 1.0 and 4.0 M brines (orange symbols, left vertical axis). Salt removal was higher for 4.0 M NaCl brine compared to 1.0 M with all amines. The enhanced salt removal at higher NaCl concentration highlights that TSSE is especially favorable for ultrahigh salinities.
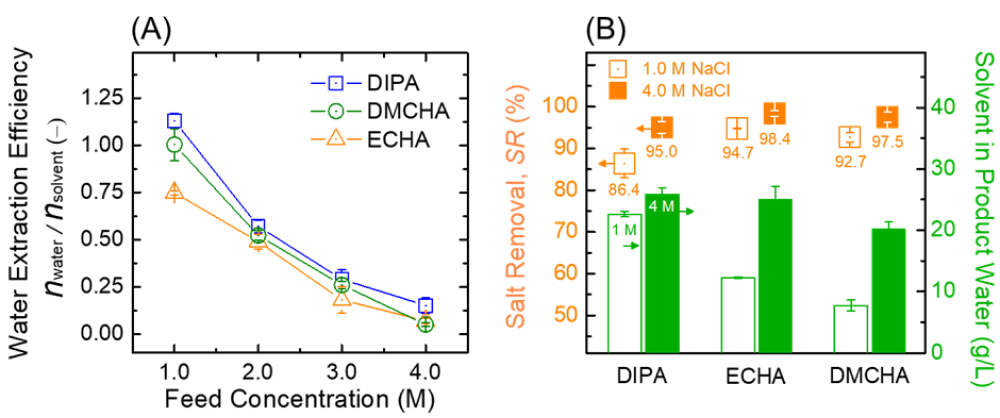
Figure 3. (A) Water extraction efficiency of DIPA, DMCHA, and ECHA as a function of brine (NaCl) concentration, expressed as the mole ratio of product water to solvent. (B) Salt removal and residual solvent concentrations in the product water (symbols, left vertical axis and columns, right vertical axis, respectively) for desalination of 1.0 M and 4.0 M NaCl brines.
-
- Wastewater management strategies that eliminate liquid waste exiting the facility are termed zero liquid discharge (ZLD). Entirely abating liquid discharge lessens environmental impacts and diminishes pollution risks. The waste solids produced in ZLD can be more easily disposed in leach-proofed landfills or further processed to recover mineral byproducts of value. Increasingly stricter disposal regulations and financial incentives are motivating the development of ZLD technologies for waste brines.
- Application of TSSE technique can be extended to achieve zero liquid discharge of ultrahigh salinity brines. The working principles of TSSE-ZLD are illustrated in Figure 4. First, the ultrahigh salinity feedwater is mixed into the solvent at temperature TL (step I). The solvent possesses hydrophilic moieties in a mainly hydrophobic chemical structure and, thus, water favorably partitions from the aqueous brine phase into the solvent, while ionic species, such as Na+ and Cl−, are retained in the brine. As the aqueous brine phase is being dewatered, salt concentrations increase and mineral solids eventually precipitate out when the solubility limit is exceeded. By using sufficient amounts of solvent relative to the brine volume, all the water can be extracted to yield only solid precipitates and a single liquid phase of the solvent, i.e., no aqueous phase. The salt precipitates are sieved off, leaving a water-laden solvent (step II). The solvent is warmed to a moderately high temperature of TH (below ≈80 °C). Because the solubility of water in the solvent decreases with an increase in temperature, the temperature swing from TL to TH drives the phase separation of desalted water from the solvent, producing a biphasic mixture (step III). The aqueous phase of desalinated product water is easily decanted from the solvent phase. The regenerated solvent is cycled back for reuse in a subsequent TSSE-ZLD cycle after returning to temperature TL (step IV).
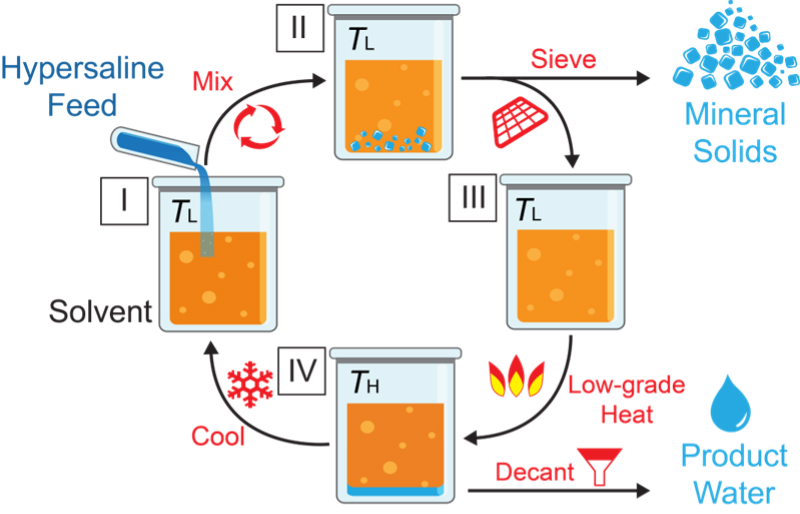
Figure 4. Schematic illustrating the working principles of temperature swing solvent extraction for zero liquid discharge of highly concentrated brines
-
- Increasing amounts of 5.0 M NaCl brine were introduced into DIPA solvent to evaluate TSSE-ZLD performance at brine to solvent ratios, ø, of 2.5, 5.1, 10.1, 15.2, 20.2, and 25.3 mL/mol. Images of the brine and DIPA mixtures at TL provide corroborative evidence of the solvent saturation results (Figure 5, with magnification of the vial bottom). For ø <15.2 mL/mol, two distinct phases comprising a single liquid layer of solvent (colored with red dye) and solid precipitates (white solids at the bottom of the vial) were observed. In contrast, once the solvent is saturated with water at ø > 15.2 mL/mol, water in the NaCl solution is unable to further partition into the solvent and, thus, an additional aqueous brine phase is seen (clear solution below solvent).

Figure 5. Photographic images of 5.0 M NaCl brine combined with DIPA solvent at increasing brine volume to solvent mole ratios
-
- We demonstrates the potential of temperature swing solvent extraction for the unprecedented zero liquid discharge treatment of hypersaline brines with extremely high total dissolved solids concentrations of ≈292,500 mg/L. Together with the findings of our previous work, where the technology was applied for the energy-efficient dewatering of high-salinity brines with up to 234,000 mg/L TDS, TSSE can be a more sustainable alternative approach for ZLD.
-
-
Chanhee Boo, Ian H. Billinge, Xi Chen, Kinnari M. Shah, and Ngai Yin Yip. “Zero Liquid Discharge of Ultrahigh Salinity Brines with Temperature Swing Solvent Extraction”, Environ. Sci. Technol. (2020), 54 (14) 9124-9131
Chanhee Boo, Robert K. Wilton, Kelly M. Conway, and Ngai Yin Yip, “Membrane-less and Non-evaporative Desalination of Hypersaline Brines by Temperature Swing Solvent Extraction”, Environ. Sci. Technol. Lett., 6 (6) (2019) 359-364
Chanhee Boo, Heyang Qi, Ian H. Billinge, Kinnari M. Shah, Hanqing Fan, and Ngai Yin Yip. “Thermomorphic Hydrophilicity Base-Induced Precipitation for Effective Descaling of Hypersaline Brines”, ACS ES&T Engineering, (2021), 1 (9) 1351-1359
-
Chanhee Boo, Ian H. Billinge, Xi Chen, Kinnari M. Shah, and Ngai Yin Yip. “Zero Liquid Discharge of Ultrahigh Salinity Brines with Temperature Swing Solvent Extraction”, Environ. Sci. Technol. (2020), 54 (14) 9124-9131



